Preparation product
Your current position:Home > Preparation product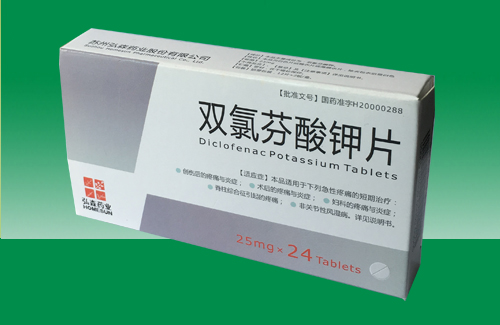
- diclofenac potassium
Diclofenac Potassium Tablets product summary
Please use it under the guidance of a doctor
[drug name]
Generic name: Diclofenac Potassium Tablets
Potassium Tablets Diclofenac
Chinese Pinyin: Pian Shuanglufensuanjia
[properties] this product is white or sugar coated tablets or film coated tablets; after the coating was removed white.
[indications]
This product is suitable for the short-term treatment of acute pain:
Post traumatic pain and inflammation, such as strain, muscle strain, etc.;
Postoperative pain and inflammation, such as dental or orthopedic surgery, etc.;
Gynecological pain and inflammation, such as primary dysmenorrhea or accessory inflammation and so on;
Pain caused by spinal syndrome;
Non articular rheumatism.
The infection of pain and inflammation of Otolaryngology (such as severe tonsillitis, sinusitis, otitis, etc.) should also use of anti infective drugs.
[Specification] 25mg
[usage] it is oral, before meals. Adult, 100 ~ 150mg / day (every 4 to 6 eggs); lesser symptoms and 14 years old children above, 75 ~ 100mg / day (every 3 ~ 4 pieces), 2 ~ 3 times to take. Primary dysmenorrhea, normal dose 50 to 150mg / day (every day 2 to 6), according to the illness can increased to a maximum dose of 200mg / day (day 8), or as directed by a physician.
[adverse reactions]
1. Occasionally upper abdominal pain and nausea, vomiting, diarrhea, abdominal cramps, indigestion, flatulence, anorexia. Rare gastrointestinal bleeding, hematemesis, melena, gastrointestinal ulcer, perforation, bleeding diarrhea.
2 I have a headache, dizziness, vertigo, rare sleep.
3 occasionally see a rash. Rare urticaria.
4 occasionally see serum aminotransferase (SGOT, SGPT) increased, rare hepatitis.
5 rare allergic reactions, such as asthma and so on.
6 rare edema.
[taboo]
1 known for this product as well as other non steroidal anti-inflammatory drug allergy disabled.
2 patients who took aspirin or other non steroidal anti-inflammatory drugs to induce asthma, urticaria, or allergic reactions.
3 disable perioperative pain treatment in coronary artery bypass surgery (CABG).
4 patients with a history of gastrointestinal bleeding or perforation after the use of non steroidal anti-inflammatory drugs.
5 there are active peptic ulcer / bleeding, or patients with recurrent ulcer / bleeding.
6 patients with severe heart failure.
7 acetyl salicylic acid or other prostaglandin synthase inhibitors cause asthma, urticaria or acute rhinitis patients disabled.
[note]
1 avoid the combination with other non steroidal anti-inflammatory drugs, including selective COX-2 inhibitors.
2 according to the needs of the control symptoms, the minimum effective dose is used in the shortest treatment time, which can minimize the adverse effects.
3 in the use of all non steroidal anti-inflammatory drugs during the treatment may occur at any time, gastrointestinal bleeding, ulcer and perforation of the adverse reaction, the risk may be fatal. These adverse reactions may be associated with or without warning symptoms, regardless of whether the patient has a history of gastrointestinal adverse reactions or serious gastrointestinal events the history of history. History of gastrointestinal disease (ulcerative colitis, Crohn's disease) patients should be cautious about the use of non steroidal anti-inflammatory drugs, so as not to make the condition worse. When patients taking the drug or ulcer gastrointestinal bleeding, the drug should be discontinued. Elderly patients with non steroidal anti-inflammatory drug adverse reaction frequency increase, especially gastrointestinal bleeding and perforation, the risk may be fatal.
4. For a variety of COX-2 selective or nonselective NSAIDs drug sustained time of 3 years of clinical trials showed, this product may cause serious adverse cardiovascular thrombotic events and increase the risk of myocardial infarction and stroke, the risk may be fatal. All NSAIDs, including COX-2 selective selective or non selective drugs, may have a similar risk. Patients with cardiovascular disease or risk factors for cardiovascular disease, the greater risk. Even if no prior cardiovascular symptoms, doctors and patients should also such events occur remain vigilant. Should inform patients with severe cardiovascular safety of symptoms and / or signs and if steps should be taken.
Patients should be wary of such as chest pain, shortness of breath, weakness, slurred speech and other symptoms and signs, and when there are any of these symptoms or signs should immediately seek medical help.
. and all non steroidal anti-inflammatory drugs (NSAIDs), this product can lead to hypertension or aggravate existing hypertension symptoms, which any one can lead to an increase in the incidence of cardiovascular events. Taking thiazide or loop diuretics in patients taking non steroidal anti-inflammatory drugs (NSAIDs) may affect the efficacy of these drugs. Patients with hypertension should be careful with non steroidal anti-inflammatory drugs (NSAIDs), including the product. In start treatment of this product and the whole course of treatment should closely monitor blood pressure.
6 have high blood pressure and / or heart failure (such as fluid retention and edema) should be used with caution in patients with a history of.
7.NSAIDs, including this product may cause fatal and severe cutaneous adverse reactions, such as peeling off dermatitis, Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (ten). These serious incidents can occur in case there is no sign of syndrome. Should be informed of the signs and symptoms of patients with severe skin reactions, in the first skin rash or allergic reaction of other signs should be discontinued this product.
. the heart, kidney function damage symptoms or history, elderly patients taking diuretics and due to any reason of extracellular fluid loss of patients with caution.
9 individual patients need long-term treatment, should regularly check the liver function and hemogram, liver damage occurs when the goods should be discontinued.
10 patients with a history of vertigo or other central nervous system should be prohibited from driving or operating the machine during the period of taking this product.
11. We should pay attention to the goods and lithium preparation, digoxin preparation, potassium sparing diuretics, anticoagulants, hypoglycemic agents and ammonia armor pterin methotrexate and used in conjunction with the dose and adverse reactions.
12. elderly patients with lower body weight and lower body weight should be reduced the amount of this product.
[pregnant women and lactating women use] this product can be through the placenta. Animal test for fetal mice are toxic, but not to be deformed.
Children under 14 years of age do not recommend the use of this product.
[old drug] this product can cause or aggravate the elderly gastrointestinal bleeding, gastric ulcer and perforation. Damage to liver and kidney function in elderly patients taking diuretics or extracellular fluid loss in elderly patients with caution.
[drug overdose drugs overdose should following treatment measures should be taken as soon as possible gastric lavage and activated carbon treatment, to prevent the further absorbed by using the. Complications, such as low blood pressure, renal failure, convulsion, stimulate the gastrointestinal, respiratory depression should be supportive treatment and symptomatic treatment.
[storage] seal in the dry place to save.
[packing] plastic packaging, 10 * 2 plate / box; 12 x 2 plate / box.
[Effective] 36 months
[executive standard] < Chinese Pharmacopoeia >2015 version two
[approval] Zhunzi H20000288




 苏公网安备 32058502010036号
苏公网安备 32058502010036号