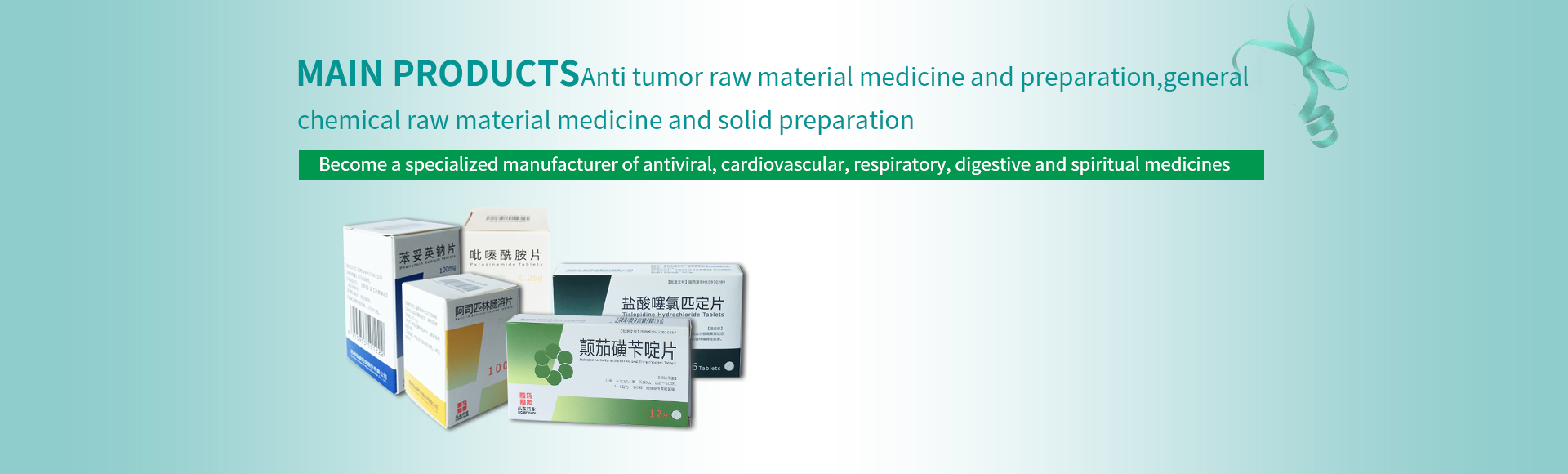
Preparation product
Your current position:Home > Preparation product
- Erythromycin
Erythromycin Enteric-coated Tablets product summary
Please use it under the guidance of a doctor.
[drug name]
Generic name: Erythromycin Enteric-coated Tablets
Enteric-coated Tablets Erythromycin
Chinese Pinyin: Changrong Pian Hongmeisu
[properties] this product is enteric coated tablet or enteric film coated tablets, after removing the film, color white or white.
[indications]
1. The product can be as patients with penicillin allergy treatment following infection of alternative medication: hemolytic streptococcus, Streptococcus pneumoniae caused by acute tonsillitis, acute pharyngitis, sinusitis; hemolytic streptococcus caused by scarlet fever cellulitis; diphtheria and diphtheriaphor; gas gangrene, anthrax, tetanus, actinomycosis; syphilis; listeriosis.
2 Corps bacterial disease.
3 Mycoplasma pneumoniae pneumonia.
4 Chlamydia pneumoniae pneumonia.
5 chlamydia, Mycoplasma caused by urinary and reproductive system infection.
6 Chlamydia conjunctivitis.
7 Neisseria gonorrhoeae infection.
8 oral infections caused by anaerobic bacteria.
9 jejunum flexure enteritis.
10 pertussis.
[Specification] 0.125g (12.5 units) 0.25g (25 units)
[usage and dosage] oral, adult day 1 ~ 2G, 3 ~ 4 times to take. The group of patients with bacterial disease, one day 2 ~ 4G, divided into 4 times. Child by weight on a day 30 ~ 50mg/kg, 3 ~ 4 times.
[adverse reactions]
1 gastrointestinal reactions are diarrhea, nausea, vomiting, epigastric pain, mouth pain, appetite loss, the incidence rate is related with the dose size.
2 rare liver toxicity, patients can have fatigue, nausea, vomiting, abdominal pain, fever and liver function abnormalities, even see jaundice.
3. High dose (equal to or more than 4G / day) applications, especially in the liver, kidney disease patients and elderly patients may cause hearing loss, and blood drug concentration is too high (>12mg/L) related to the withdrawal of most return.
4 allergic reactions showed drug fever, skin rash, eosinophils and so on, the incidence of about 0.5% ~ 1%.
I have 5 other, arrhythmia, oral or vaginal Candida infection.
[taboo] to this product and other big ring of the allergy of the medicine is forbidden.
[note] [note]
1 hemolytic streptococcus infection in the treatment of this product, at least 10 days to continue to prevent the occurrence of acute rheumatic fever.
2 patients with impaired renal function generally do not need to reduce the dosage, but the dose of the patients with severe renal impairment should be reduced appropriately.
3 the dosage of this product should be appropriately reduced in patients with liver diseases.
4 regular follow-up liver function.
5 patients who are allergic to or can not bear the erythromycin preparation, the other erythromycin preparation may also be allergic or can not afford.
6 because there are some differences in the sensitivity of different bacteria to erythromycin, it should be sensitive to drug sensitivity test.
7 the interference of diagnosis: This product can interfere with the fluorescence measurement of Higerty method, so that the determination of urinary catecholamine increased false; serum alkali
Phosphatase, bilirubin, alanine aminotransferase and aspartate aminotransferase were all increased.
[pregnant women and breastfeeding women medication]
This product can enter the fetal circulation through the placenta barrier, so pregnant women should be used with caution.
This product has a considerable amount into breast milk, so the lactating women should be used with caution or suspend breastfeeding.
The experiment was not carried out and there was no reliable references.
The experiment was not carried out and there was no reliable references.
The experiment was not carried out and there was no reliable references.
[storage] seal in the dry place to save.
[packing] plastic bottle packaging, 100 / bottle.
[Effective] 36 months
[executive standard] < Chinese Pharmacopoeia >2015 version two.
[approval] Zhunzi H32022516




 苏公网安备 32058502010036号
苏公网安备 32058502010036号